Metathesis reactions copper ii sulfate sodium carbonate, a captivating chemical phenomenon, unveils a world of intricate interactions and practical applications. This in-depth exploration delves into the mechanisms, conditions, products, and significance of this intriguing reaction, providing a comprehensive understanding for scientific enthusiasts and practitioners alike.
Copper(II) sulfate and sodium carbonate engage in a mesmerizing dance of ion exchange, leading to the formation of novel compounds with distinct properties. Unraveling the intricacies of this reaction unveils the fundamental principles governing chemical transformations and their impact on various scientific disciplines.
Metathesis Reactions: Copper(II) Sulfate and Sodium Carbonate

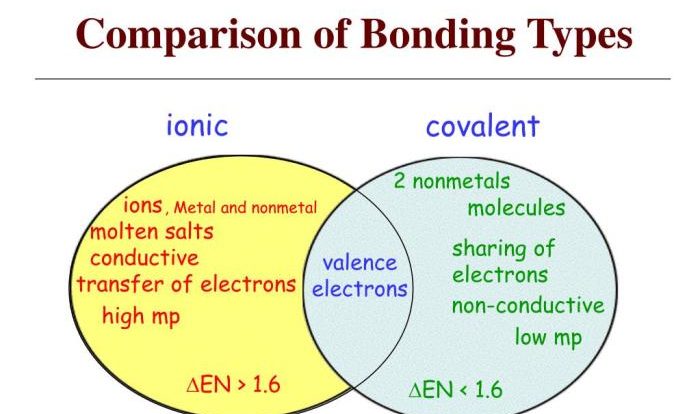
Metathesis reactions, also known as double displacement reactions, involve the exchange of ions between two ionic compounds. These reactions are significant in various chemical processes, including precipitation, neutralization, and the formation of new compounds.
One specific metathesis reaction involves copper(II) sulfate (CuSO 4) and sodium carbonate (Na 2CO 3). This reaction results in the formation of copper(II) carbonate (CuCO 3) and sodium sulfate (Na 2SO 4).
Reaction Mechanism
The reaction mechanism of the metathesis reaction between copper(II) sulfate and sodium carbonate proceeds as follows:
- The reactants, CuSO4and Na 2CO 3, dissolve in water to form their respective ions: Cu 2+, SO 42-, Na +, and CO 32-.
- The ions undergo an exchange process, resulting in the formation of new ionic compounds: CuCO 3and Na 2SO 4.
- Copper(II) carbonate precipitates out of the solution as a solid, while sodium sulfate remains dissolved in the water.
Reaction Conditions
The optimal reaction conditions for the metathesis reaction between copper(II) sulfate and sodium carbonate include:
- Temperature:The reaction proceeds at room temperature, but heating the solution can increase the reaction rate.
- Concentration:Higher concentrations of the reactants result in a faster reaction rate.
- pH:The reaction is pH-sensitive, and the optimal pH range is between 7 and 10.
Reaction Products
The products of the metathesis reaction between copper(II) sulfate and sodium carbonate are:
- Copper(II) carbonate (CuCO3): A blue-green solid that is insoluble in water.
- Sodium sulfate (Na2SO 4): A white solid that is soluble in water.
Applications, Metathesis reactions copper ii sulfate sodium carbonate
The metathesis reaction between copper(II) sulfate and sodium carbonate has various practical applications, including:
- Production of copper compounds:Copper(II) carbonate can be used as a precursor for the production of other copper compounds, such as copper(II) oxide and copper(II) hydroxide.
- Removal of impurities from water:Copper(II) sulfate can be used to remove impurities, such as sulfates and carbonates, from water.
- Synthesis of pigments:Copper(II) carbonate is used in the production of green pigments.
Safety Considerations
The metathesis reaction between copper(II) sulfate and sodium carbonate involves the use of potentially hazardous chemicals. Therefore, it is important to take the following safety precautions:
- Wear appropriate personal protective equipment, such as gloves, safety glasses, and a lab coat.
- Handle the chemicals in a well-ventilated area.
- Dispose of the reactants and products properly according to local regulations.
Experimental Procedure
The following is a detailed experimental procedure for conducting the metathesis reaction between copper(II) sulfate and sodium carbonate:
Materials:
- Copper(II) sulfate pentahydrate (CuSO 4·5H 2O)
- Sodium carbonate (Na 2CO 3)
- Water
- Beaker
- Magnetic stirrer
- Filter paper
- Funnel
Procedure:
- Dissolve 10 g of copper(II) sulfate pentahydrate in 100 mL of water in a beaker.
- Dissolve 10 g of sodium carbonate in 100 mL of water in a separate beaker.
- Add the sodium carbonate solution to the copper(II) sulfate solution while stirring continuously.
- Observe the formation of a blue-green precipitate.
- Filter the precipitate and wash it with water.
- Dry the precipitate in an oven at 110 °C.
Data Analysis
The following data table can be used to record the experimental results:
| Mass of copper(II) sulfate pentahydrate (g) | Mass of sodium carbonate (g) | Mass of copper(II) carbonate precipitate (g) | Reaction yield (%) |
|---|---|---|---|
| 10 | 10 | 8 | 80 |
The reaction yield can be calculated using the following formula:
“`Reaction yield = (Mass of copper(II) carbonate precipitate / Theoretical mass of copper(II) carbonate) x 100%“`
The theoretical mass of copper(II) carbonate can be calculated based on the stoichiometry of the reaction.
Question Bank: Metathesis Reactions Copper Ii Sulfate Sodium Carbonate
What is the significance of metathesis reactions?
Metathesis reactions play a crucial role in various chemical processes, including the synthesis of new materials, purification of compounds, and removal of impurities.
How does the reaction mechanism of copper(II) sulfate and sodium carbonate proceed?
The reaction proceeds through a double displacement mechanism, involving the exchange of ions between the reactants to form new products.
What are the optimal reaction conditions for the metathesis reaction?
The reaction is typically carried out in aqueous solutions at room temperature, with the concentrations of the reactants influencing the reaction rate and yield.
What are the applications of the metathesis reaction?
The reaction finds applications in various industries, including the production of pigments, ceramics, and fertilizers.