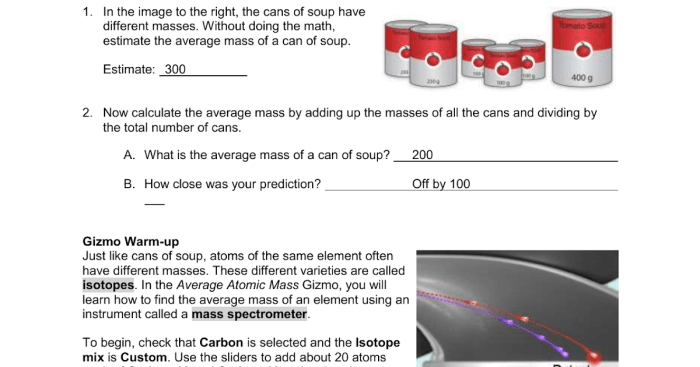
The POGIL Average Atomic Mass Answer Key unlocks the mysteries of chemistry, providing a comprehensive guide to understanding this fundamental concept. This answer key is an essential tool for students seeking to master the intricacies of atomic mass and its applications in various chemical calculations.
This meticulously crafted answer key not only provides accurate solutions but also offers insightful explanations that foster a deep understanding of the subject matter. It serves as an invaluable resource for students, educators, and anyone eager to delve into the fascinating realm of chemistry.
Average Atomic Mass: Pogil Average Atomic Mass Answer Key

Average atomic mass is a weighted average of the masses of all the naturally occurring isotopes of an element, taking into account their relative abundances.
It is an important concept in chemistry because it allows us to determine the mass of an element in a compound and to perform stoichiometric calculations.
Determining Average Atomic Mass
The periodic table is organized by atomic number, which is the number of protons in the nucleus of an atom.
The atomic mass of an element is listed below the element’s symbol in the periodic table.
To calculate the average atomic mass of an element, we need to know the isotopic composition of the element.
- Multiply the mass of each isotope by its relative abundance.
- Add up the products from step 1.
- Divide the sum from step 2 by 100.
Applications of Average Atomic Mass
Average atomic mass is used to determine the molar mass of compounds.
The molar mass of a compound is the mass of one mole of the compound.
Average atomic mass is also used in stoichiometric calculations.
Stoichiometric calculations are used to determine the amount of reactants and products in a chemical reaction.
Limitations of Average Atomic Mass
Average atomic mass does not take into account the natural abundance of isotopes.
The natural abundance of isotopes can vary from sample to sample.
This can lead to variations in the average atomic mass of an element.
Using the POGIL Activity, Pogil average atomic mass answer key
The POGIL (Process Oriented Guided Inquiry Learning) activity is a student-centered learning activity that can be used to teach students about average atomic mass.
The POGIL activity can be implemented in the classroom by following these steps:
- Introduce the concept of average atomic mass.
- Have students work in groups to complete the POGIL activity.
- Discuss the results of the POGIL activity with the class.
General Inquiries
What is the significance of average atomic mass?
Average atomic mass plays a crucial role in determining the molar mass of compounds and is essential for stoichiometric calculations.
How does the POGIL activity enhance the learning of average atomic mass?
The POGIL activity engages students in hands-on exploration, fostering a deeper understanding of atomic mass and its applications.